Ah, I think I’m tracking now….I thought you were saying 84 pounds of P and K when I first read it but you are saying 84 pounds of 6-28-28 of which will equate to an application of around the 21 pounds of each that I need. I think anyway? Haha.
Think i read that the sulfur/sulfate will lower the soil ph….is that true and if so is that a concern? Or is that why id also add the lime at same to essentially counteract and make it a wash.
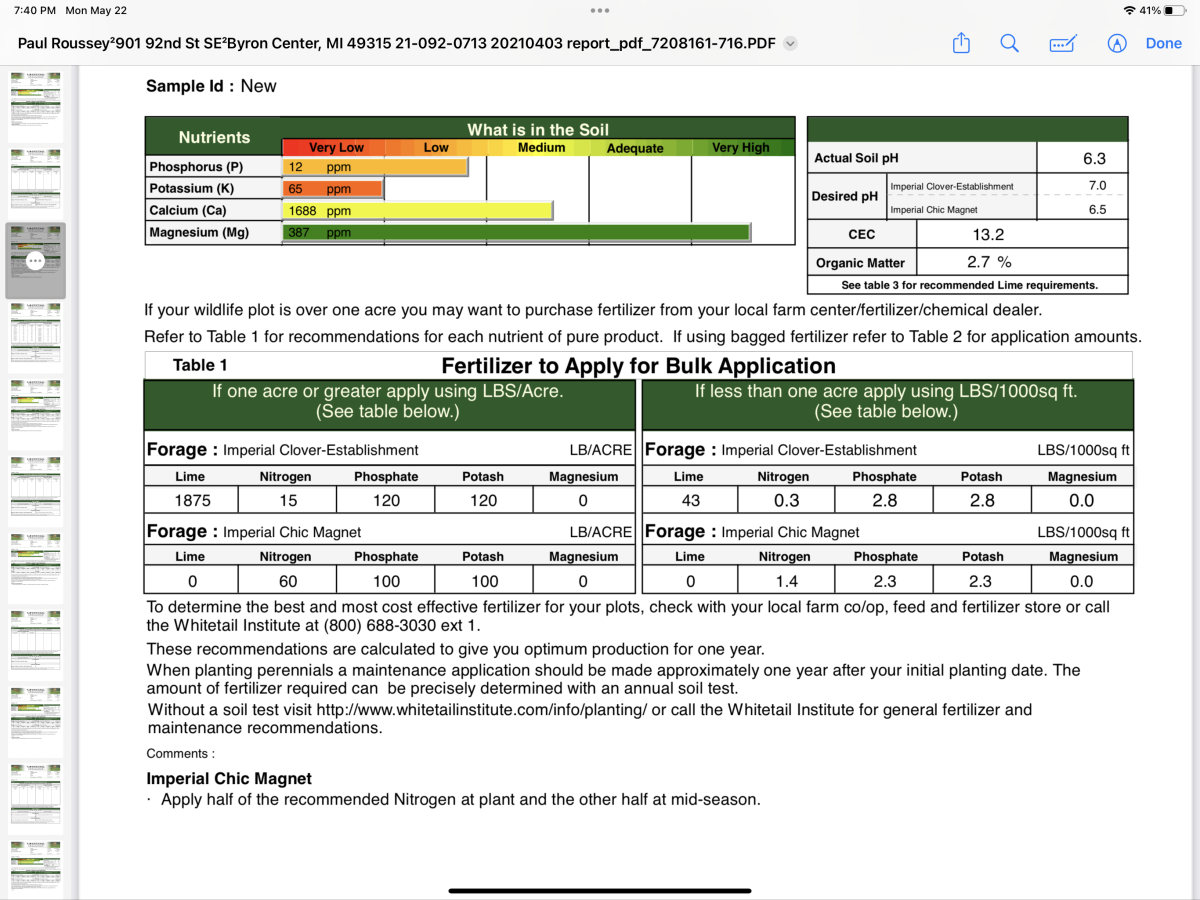
Also, when it comes to p and k, other than wasted expense does it do any harm to a plot if too much were to get applied as long as we’re not talking crazy excessive?
great information, thanks a bunch for the explanation!
I am saying with a single 50# bag of 6-28-28, you would be applying 18# of actual N, 84# P, and 84# K to your 1/6 acre area.
Deep dive into soil structure and fertility:
Compared to Calcium, magnesium will
elevate pH by 1.65X versus lime. Soils that tend to be higher in magnesium (mine is identical to yours) give a
false indication of soil fertility by showing an elevated pH. So, your pH can be adequate (6.2+ for example), but you still could have a calcium deficiency.
Typically, Calcium below 60% base saturation is not ideal. Until you get a better soil test showing actual base saturation percentages (From Midwest Labs, etc), you'll never know for sure.
Pure sulfur/sulfate will lower pH by bonding with magnesium and dropping out of the soil (cation / anion relationship). Since magnesium is 1.65X more effective at elevating pH than lime, you can lose pH with pure sulfur/sulfate. The calcium in calcium sulfate can even out the process however, so typically gypsum doesn't move the soil pH meter much.
Adding high Ca / low Mg lime will right the ship, increase pH and get you to a better soil
structure and balance overall. Yes, your pH will be a "wash" but you will have a much better physical soil structure, which lends itself to better microbial growth, moisture management and overall soil fertility.
Think of Calcium and Magnesium as a teeter totter in the soil. Elevating calcium by one percent (base saturation), will drop magnesium by one percent - And vice versa. Calcium makes the soil more porous (good if you have tight soils), and magnesium tightens the soil (good if your calcium is too high and your soil doesn't hold moisture).
Excessive amounts are not the best practice. In short terms - Any excess of one nutrient pushes out another. As the soil colloid (dinner plate) can only hold so much (food), before it is lost by dropping out of the top layer.